- 19/1/16
- 4,412
- 5,075
- 113
Amazing work! Congratulations! Love the green and gold combination
Thank you
Amazing work! Congratulations! Love the green and gold combination
Hey buddy, i wear proper gas mask and my solutions don't have cyanide as far as i know. I don't use nickel as it's highly toxic and a lot of people are allergic.
I keep each part around 12-13 minutes for heavy gold plate.
Thank you !
Naw it's Cyanide.
You have to use Nickle though for substrate since it's a stainless steel watch. Just copper will migrate through the gold plating without Nickel. It wouldn't be a harmful release of 0.5ug/cm2/week.
The long baths only works if you maintain a temperature and PH within a 2-3% margin of error, which at that point becomes an art work. Also 1 litre of gold solution is like 400usd which is supposed to give 2.5 microns. 2-3min in a bath gives you 1 micron. So with 12-13min you would need easily over 1kusd of solution.
If you put each part in the solution for 140 seconds, then rinse in distilled and repeat a few times it's the best way to achieve a proper finish with 1 litre of solution. Then you can UV coat for more protection if you want. All this stuff doesn't matter though if you don't use Nickle.
My first time I made it too red... Expensive experience haha. It's expensive but fun lol.
Naw it's Cyanide.
You have to use Nickle though for substrate since it's a stainless steel watch. Just copper will migrate through the gold plating without Nickel. It wouldn't be a harmful release of 0.5ug/cm2/week.
The long baths only works if you maintain a temperature and PH within a 2-3% margin of error, which at that point becomes an art work. Also 1 litre of gold solution is like 400usd which is supposed to give 2.5 microns. 2-3min in a bath gives you 1 micron. So with 12-13min you would need easily over 1kusd of solution.
If you put each part in the solution for 140 seconds, then rinse in distilled and repeat a few times it's the best way to achieve a proper finish with 1 litre of solution. Then you can UV coat for more protection if you want. All this stuff doesn't matter though if you don't use Nickle.
My first time I made it too red... Expensive experience haha. It's expensive but fun lol.
You’re not updated: there are cyanide free solutions all over the market.
We also have available nickel free flash baths made to stick on stainless and other hard to plate materials and prepare them for the subsequent thick plating bath, and there’s gold in it instead of nickel. Nickel woods technique is outdated and only required in specific circumstances.
edit: normally (at least on my solutions) we have 4 grams of gold per liter. That’s way more than 2.5 microns of deposit on a watch.
You’re not updated: there are cyanide free solutions all over the market.
We also have available nickel free flash baths made to stick on stainless and other hard to plate materials and prepare them for the subsequent thick plating bath, and there’s gold in it instead of nickel. Nickel woods technique is outdated and only required in specific circumstances.
edit: normally (at least on my solutions) we have 4 grams of gold per liter. That’s way more than 2.5 microns of deposit on a watch.
The Cyanide free solutions are not popular with rings and watches for a reason. Contact resistance is similar to Cyanide solutions however wear resistance is where it becomes flawed.
Cyanide free is more for chains, broaches and pendants because they don't suffer from high wear like watches and rings.
Lots of studies have been conducted using MFG tests for 5 days under 70% humidity/ 30°C as well as exposure to NAV testing for a few hours.
Cyanide free solution produced very poor wear resistance during the test.
It shined best in corrosion resistance obviously though. The conclusion was to add higher Nickle content to match wear resistance of Cyanide solutions.
Nickel Woods is far from being outdated because it offers the best protection. If Europeans were allowed to use it they would. Since the ban, European jewelers had to find alternatives like white bronze but it never offered the same quality Nickle strikes did.
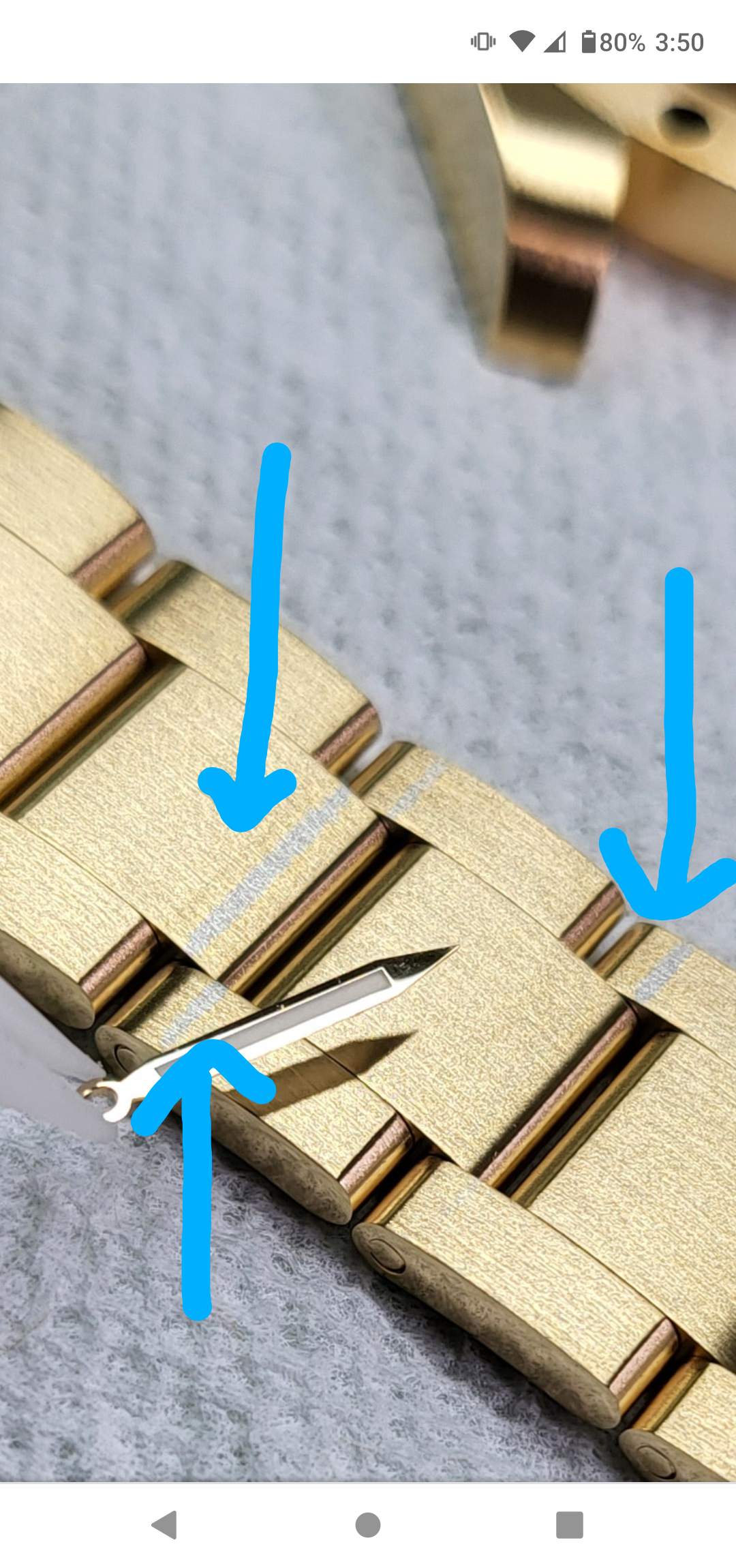
Also 904L has 25% nickle. Soon as you put the parts in the bath the solution is contaminated with Nickle so it's definitely not Nickle free. The other issues with not using nickle on stainless steel is the matte finish it produces which I pointed out in my Screenshot.
Last points, I'm sure you know from experience that you never get the full 4 grams of pure gold listed by the manufacturer unfortunately. There is always solution loss when in use. Cyanide free or not once the pH levels drop low the gases get released which is why detectors and proper ventilation is a must.

The Cyanide free solutions are not popular with rings and watches for a reason. Contact resistance is similar to Cyanide solutions however wear resistance is where it becomes flawed.
Cyanide free is more for chains, broaches and pendants because they don't suffer from high wear like watches and rings.
Lots of studies have been conducted using MFG tests for 5 days under 70% humidity/ 30°C as well as exposure to NAV testing for a few hours.
Cyanide free solution produced very poor wear resistance during the test.
It shined best in corrosion resistance obviously though. The conclusion was to add higher Nickle content to match wear resistance of Cyanide solutions.
Nickel Woods is far from being outdated because it offers the best protection. If Europeans were allowed to use it they would. Since the ban, European jewelers had to find alternatives like white bronze but it never offered the same quality Nickle strikes did.
Also 904L has 25% nickle. Soon as you put the parts in the bath the solution is contaminated with Nickle so it's definitely not Nickle free. The other issues with not using nickle on stainless steel is the matte finish it produces which I pointed out in my Screenshot.
Last points, I'm sure you know from experience that you never get the full 4 grams of pure gold listed by the manufacturer unfortunately. There is always solution loss when in use. Cyanide free or not once the pH levels drop low the gases get released which is why detectors and proper ventilation is a must.

Ahem. Acidic cyanide free solutions are for the flash gold baths (which replaces nickel) then after it you’re just plating over a layer of 18k gold.
I personally never had the effect you pointed in your screenshot, not even once. And I don’t use nickel.
Nikz19 has to comment on that i only fallow his guidance as he's expert in my eyes.
Picture you pointed wire was touching that's why I said I'm practicing. I was testing color on hands vs bracelet it's been plated later.
I'm all about learning but i will never use nickel.
Ahem. Acidic cyanide free solutions are for the flash gold baths (which replaces nickel) then after it you’re just plating over a layer of 18k gold.
I personally never had the effect you pointed in your screenshot, not even once. And I don’t use nickel.
I'm not really following you? The pure gold Cyanide free solution just doesn't magically adhere to the surface you are plating. It needs to be prepared. Even more so with Stainless steel. Just cleaning is not enough for SS.
If you are using the exact same solutions oascom is using and it's not producing that matte finish then you are doing a thinner layer maybe, the matte finish happens when you apply too thick of cyanide free gold solution with no nickle . How long do you keep it in the solution? Maybe explain your process for plating a bracelet link? Here in North America cyanide free gold is not used for jewelry but more for medical equipment. I know you guys have a bunch of different rules in place there.
Sure it’s a thinner layer. 4 steps:
Degreasing-activator-flash-thick plating.
The activator is what makes the gold “magically” adhere
I don’t use the same solutions Pat use, but I think they’re similar.
And yeah, we have different regulation than US here. Safety first ehehs
Ohhh okay I get what your saying. You use gold strike instead of Nickel Strike and then cyanide free solutions. Yea personally I would never gold strike on stainless steel unless it's for medical tools that require it for safety reasons.
You would never use it with jewelry, especially watches where I think you would agree wear resistance is very important.
Sure gold strike will allow the gold to be DEPOSITED however adhesion is very inconsistent. The proper and most effective method is with Nickel Strike. Gold strike is an ALTERNATIVE method.
Is oascom in Europe or USA? I know he said he didn't want to use Nickel Strike in general but it's such a thin layer and will provide excellent results and has no effect on the person's skin unless some members like to put their watches in their mouth hahaha.
Quite the opposite! The cyanide free solutions I use are patent pending and made specifically for use on precious metals only. If used on a nickel layer, they won’t adhere as supposed and I should use different solutions.
The deposited layer can actually be safely brushed/scratched with a nail. It’s quite resistant.
That all depends by exposition times ofc
Considering how many platings I’ve done in the past 2 years if it was crap, you would see disappointed comments all over the forums
